KesimptaⓇ (ofatumumab): A new treatment for relapsing-remitting multiple sclerosis (RRMS)
Published May 6, 2021 • By Aurélien De Biagi
Following a decision from the EMA (European Medicines Agency) and the UK's MHRA (Medicines and Healthcare Products Regulatory Agency) in April 2021, Novartis is authorized to market its new drug, KesimptaⓇ, in the EU and UK . This new monoclonal antibody has already been approved by the FDA in August 2020 and is used in the treatment of relapsing remitting multiple sclerosis (RRMS).
Want to know more about this treatment? How does it work? What is a monoclonal antibody?
We explain everything in our article!
What are monoclonal antibodies?
Ofatumumab is the active ingredient in KesimptaⓇ. It is also a monoclonal antibody.
Antibodies are proteins produced by the cells of our immune system (specifically plasma cells, a type of B lymphocyte).
These proteins can bind specifically to bacteria, viruses, cancerous cells or a vaccine (an antibody will be specific to a bacterium for example). Once the antibody binds with its target, it can signal to other immune cells which can then eliminate it, prevent it from reproducing or even destroy the target cell itself.
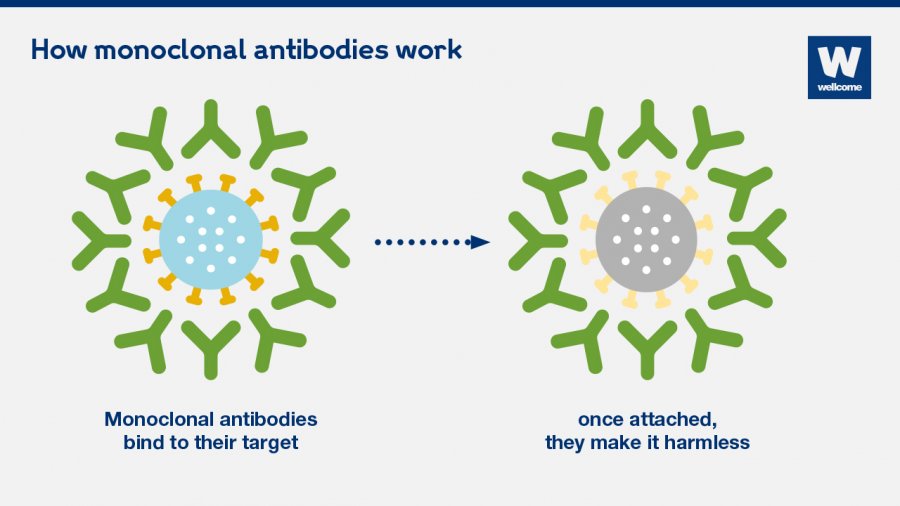
Source: The Wellcome Trust
However, these proteins do not only bind to the body's own cells (the non-self or exogenous cells). In fact, in autoimmune disorders (psoriasis, MS, etc.), the immune system mistakenly attacks the self cells, thus creating inflammation. This attack involves, among other things, the use of antibodies directed against endogenous cells.
In addition, during an organ transplant, for example, the immune system will consider the new organ as a foreign element and produce antibodies directed against it. This is why immunosuppressants are prescribed following a transplant, in order to limit this immune reaction.
Monoclonal antibodies (mAbs or moAbs) are based on natural antibodies and are produced specifically to fight a disease.
They are biotherapies, meaning that they are not produced by a chemical reaction but by living cells. Because of their size, proteins are extremely complex molecules to produce artificially. This is why living cells must be involved.
Without going into too much detail about a complex system, the production of monoclonal antibodies involves selecting a cell (bacteria, yeast, human cell, etc.) capable of producing the desired antibody. It will then be exposed to the target protein against which it will secrete an antibody. These cells are then cultured and used to produce our mAb.
Because of their proteic nature, mAbs cannot be taken orally (they are destroyed by digestive juices). They are therefore injected subcutaneously or intravenously depending on the treatment.
What is KesimptaⓇ (ofatumumab)?
As a reminder, in multiple sclerosis, the immune system attacks the myelin sheath, thus disrupting the transmission of information. KesimptaⓇ has received marketing authorization for the treatment of relapsing-remitting MS.
If you'd like to learn more about MS, you can read our fact sheet on the subject.
The CD20 antigen (a surface protein) is the target of ofatumumab antibodies. This antigen is present on the surface of the majority of B lymphocytes (immune cells) and is a marker for them. Indeed, this antigen is expressed from the pre-lymphocyte B stage to the mature lymphocyte stage. It is also present on B cancer cells (ofatumumab has been marketed in the US since October 2009 under the name Arzerra for the treatment chronic lymphocytic leukemia (CLL)).
By binding to its target, the monoclonal antibody can lyse (destroy) the immune cells. This destruction is mainly done by the complement system. This complement system, also known as the complement cascade, is a part of the innate immunity (first line of defense) and links it to the adaptive immunity (the second line).
When activated, the complement system engulfs the target pathogens or cells, which then leads to phagocytosis (absorption and destruction) of the target by other immune cells, such as macrophages. Innate immune cells are also involved.
We will now examine why this treatment has been granted marketing authorization by a brief analysis of the Phase III trials conducted
The ASCLEPIOS trials
The efficacy of this ofatumumab was evaluated in two randomized, double-blind, controlled Phase III trials (ASCLEPIOS I and II) versus a comparator (teriflunomide). Indeed, among the different phases of clinical trials, phase III studies are generally conducted on large patient populations and aim to measure the efficacy of the treatment against the reference treatment or a placebo.
In a double-blind study, neither the patient nor the administrator knows which treatment is being administered (neither knows whether the reference treatment, a placebo or the new treatment is being administered). The double-blind design also limits bias (prejudice about efficacy or side effects).
Randomization refers to the random allocation of groups. There is a random draw to determine who receives the placebo or reference treatment and who receives the new treatment.
Both studies demonstrated the efficacy of KesimptaⓇ. Indeed, it was found that out of the 1,882 patients in the studies, patients treated with ofatumumab had, over one year, half as many relapses as those treated with the reference treatment. In addition, only 8% of patients had a worsening of symptoms lasting more than 6 months compared to 12% with the reference treatment.
In addition, the adverse events recorded were in line with and considered manageable compared to the effects induced by treatments already on the market. The FDA therefore judged the benefit-risk balance in favor of the benefits. KesimptaⓇ therefore received a marketing authorization that is valid in the US since August 20, 2020.
Dosage and route of administration
This biotherapy is available in pre-filled pens or syringes. Treatment starts with a weekly injection under the skin for three weeks, followed by a week without administration. The next dose is taken the following week and then once a month.
In addition, the pre-filled pen or syringe form allows patients to self-administer the treatment at home. This represents a novelty, making this treatment the one and only self-injectable monoclonal antibody targeting B lymphocytes.
Was this article helpful to you?
Share your thoughts and questions with the community in the comments below!
Take care!
Sources:
- Kesimpta, EMA
- Les anticorps monoclonaux, Vidal
- Arzerra, Vidal
- Anticorps monoclonal anti-CD20 (rituximab) dans les maladies hématologiques et les affections auto-immunes, Science Direct
- Résumé des caractéristiques du produit, EMA
- Kesimpta (ofatumumab), EMA
- Système du complément, anticorps-enligne.fr
- What are monoclonal antibodies – and can they treat Covid-19?, The Wellcome Trust
- FDA approves Novartis Kesimpta® (ofatumumab), the first and only self-administered, targeted B-cell therapy for patients with relapsing multiple sclerosis, Novartis
- FDA Approves Kesimpta® (ofatumumab), Similar to Ocrevus®, for Relapsing MS, National MS Society

 Facebook
Facebook Twitter
Twitter


