What is an observational study and what is its purpose?
Published Jul 3, 2021 • By Clémence Arnaud
Clinical research is a very important part of the development and post-marketing monitoring of medicines and medical devices. There are many different types of studies that can be carried out depending on the study's objectives.
What is an observational study? When is an observational study conducted? What types of non-interventional studies does Carenity conduct?
We tell you everything in our article!

What is an observational study?
Observational studies are the opposite of experimental (intervention) studies. They can therefore also be called non-interventional studies. In these studies, the investigator measures the exposure to the factor being studied and the strength of its association with the event or disease being studied.
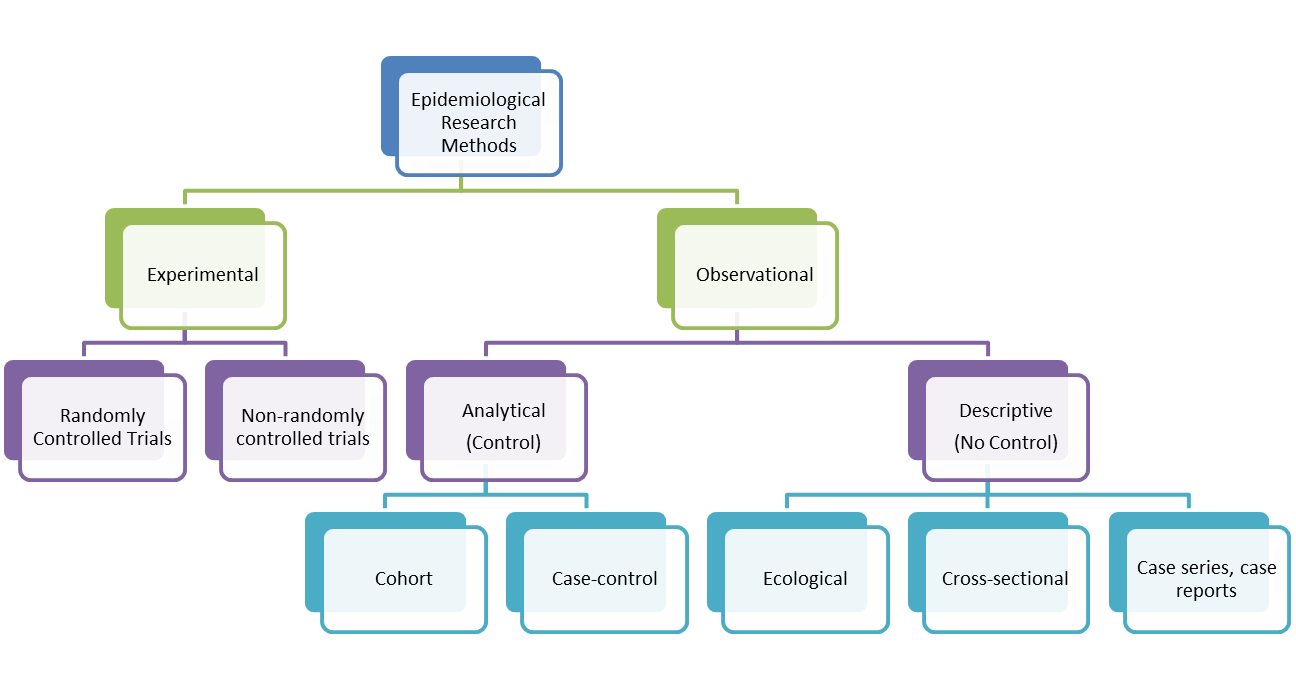
Source: Dr. Catherine Heffernan
Depending on the intended objective, there are two types of observational studies. The first is the descriptive study, which is limited to describing the frequency of exposure to the factor studied and/or the frequency of occurrence of the disease or event studied. The second type of study is the analytical study which measures the association between exposure to a factor and the occurrence of an event.
Non-interventional studies are risk-free. They do not change the care of participants, and all procedures and products used are routine. Observational research includes:
- Studies of treatment adherence,
- Studies of the tolerance of a drug after it has been put on the market,
- Studies comparing the practices of one health care center with those of another,
- Etc.
An observational study can work on patient-related data, work on biological samples or follow a cohort of patients over a given time.
How are observational studies run?
In all cases, observational research must:
- Be carried out by a sponsor who manages the study, ensures compliance with good practices guaranteeing the integrity of the study and verifies that funding has been obtained
- Have obtained approval from an institutional review board (IRB) (also called an independent ethics committee (IEC))
- Receive approval from the Food & Drug Administration (FDA) after demonstrating that the research protocol falls in line with guidelines concerning the processing of personal data.
In all clinical studies, an investigator will direct and verify the patients' understanding of the study, the data used, the objectives of the study, etc.
Observational studies on Carenity
Carenity is actively involved in clinical studies with the aim of making patients' voices heard. Collaborations with public, private and institutional health actors are carried out to improve and understand patients' needs.
In 2021, we conducted a study on psychological distress during the first COVID-19 lockdown. This study was conducted in partnership with IRDES (Institut de Recherche et Documentation en Economie de la Santé), the French Institute for Research and Information in Health Economics, and looked for specific vulnerability factors for people living with a chronic illness or disability.
A number of vulnerability factors were identified that are common among the general population. Compounded with these factors are issues related to the patient's illness or disability, such as difficulty implementing health precautions, cessation or reduction of routine medical or medicosocial care and follow-up during lockdown periods, among others. These findings underline the importance of taking into account the needs of people living with a chronic illness or disability in this particular public health crisis and especially when implementing lockdown policies.
In 2020, Carenity also conducted observational studies in various conditions such as psoriasis, asthma and diabetes. One study was undertaken to understand the reasons for poor adherence or discontinuation of treatment in the US and UK. The survey was carried out using an online questionnaire which was directed at patients who had discontinued their treatment in the 6 months prior to the study. The most common reasons for discontinuing treatment were adverse events, problems with treatment efficacy and the cost of treatment for patients in the US. Solutions such as better explaining treatments to patients, increasing the frequency of doctor-patient discussions and supporting the patient in managing treatment were identified as possible solutions to help improve treatment adherence.
Overall, the study highlighted the important role therapeutic education plays in patients' understanding of their treatments and medications and therefore in their compliance with it.
Conclusion
In summary, observational or non-interventional studies are designed to be conducted without altering the patient's daily life. They allow us to study various factors that may have an influence on both patients' condition and care. These studies are crucial for gathering and understanding the patient perspective on their disease and their treatments, an important key to advancing medical research and improving their care!
Was this article helpful to you?
Share your thoughts and questions with the community in the comments below!
Take care!
Sources:
- INSERM - Comprendre la recherche clinique
- Gainotti, Sabina & Gambino, Alberto & Greco, Donato & Gussoni, Gualberto & Mannelli, Chiara & Morresi, Assuntina & Nicoli, Federico & Resta, Giorgio & Petrini, Carlo. (2020). Research ethics during the COVID-19 pandemic: observational and, in particular, epidemiological studies ISS COVID-19 Bioethics Working Group. 10.13140/RG.2.2.31032.98569.
- Université de Lorraine - Etudes expérimentales
- CHU Grenoble Alpes - Les différents types de recherche clinique
- IRDES - Détresse psychologique pendant le premier confinement lié à la Covid-19 : des facteurs de vulnérabilité spécifiques aux personnes vivant avec une maladie chronique ou un handicap
- Springer Link - Understanding Reasons for Treatment Discontinuation, Attitudes and Education Needs Among People Who Discontinue Type 2 Diabetes Treatment: Results from an Online Patient Survey in the USA and UK
Comments
You will also like

What are the dangers associated with the over-the-counter sale of certain medicines?
Dec 19, 2020 • 6 comments

 Facebook
Facebook Twitter
Twitter

