What are the different types of clinical trials?
Published Apr 24, 2021 • By Carenity Editorial Team
Depending on the condition and the drug in development, clinical trials will aim to highlight different parameters.
But what are the different clinical trials that can be carried out today before and after the commercial release of a drug?
We tell you everything in our article!

The development of a medical product is a long and complex process. The medicine or device in question undergoes various studies before it can be put onto the market. These studies are divided into 3 phases: the research phase, the development phase, during which preclinical and clinical studies are carried out, and then the marketing phase with the product's launch on the market and its monitoring in real time. After the product has been launched, various studies can be carried out, as is the case on Carenity!
Research involving humans
The carrying out of a clinical study is based on respect for the human being. There are three categories of research involving humans:
- Interventional research (also called clinical trials): this type of research focuses on a new drug or medical device. For example, it could be a study of a new compound.
- Observational or non-interventional research: this type of research does not involve any risk to the individual and is carried out by monitoring the patient using the product in a normal way. These types of studies consist of observing the relationship between certain factors and health events. In no way is it an experimental study. An example of such a study would be: examining the incidence of lung cancer in smokers and non-smokers.
- Qualitative research: This type of research aims to understand the patient experience and relevant issues. In this type of research no products or drugs are tested, but the patient may be asked questions about his or her opinions, experiences, issues, etc.
Types of trials in interventional research
Before the start of the trial, patients will be split into different groups, either at random, or not. In other words, patients are randomly assigned to a group, or they are assigned to a group based on their treatment.
In interventional research the trial may compare:
- A new medical product to a standard one, if the study objective is to evaluate one drug against another
- A new medical product to a placebo, which is a substance that contains no active ingredients that may have a psychological effect on the patient, if the aim is to evaluate the drug's properties
- A new medical product with no intervention.
The trial design will be made according to the research plan or protocol created by the investigators, which is based on the study objectives.
How is an interventional trial run?
Parallel trial: Patients are randomly assigned to different groups and are monitored throughout the trial in the same group.
This type of trial can be conducted to compare one drug and a placebo or two drugs. The same drug can also be compared in groups with different factors (e.g. males and females, etc.).
The groups will then be compared to each other.
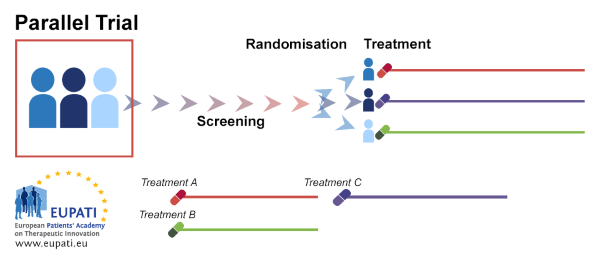
Source: European Patients' Academy on Therapeutic Innovation
Cross-over trial: In a cross-over trial, the groups receive each treatment in succession. Group A will start with treatment A and group B with treatment B in the first phase of the trial, and then in the second phase group A will receive treatment B and group B will receive treatment A.
The treatment order is randomized. For this trial each patient will be their own control.
However, these trials can be lengthy because there is a "washout" period between the two phases, i.e. a period of time during which the patient does not receive any treatment.
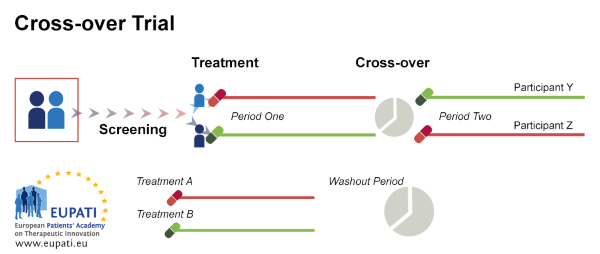
Source: European Patients' Academy on Therapeutic Innovation
Sequential trials: In this type of trial, the patients receive a first treatment and then a second treatment with a washout treatment in between. The difference is that here there will be no comparison between group A and group B.
There is also another important concept to understand when talking about clinical trials. Depending on the type of trial and its design, it is said to be conducted:
- Unblinded (or "open label"): This means that both the investigator and the patient are aware of the treatment received. However, this can lead to errors when analyzing the results. If a patient is told that he or she is taking a placebo, the patient will immediately think that no results are possible. The same applies to the interpretation of results if the investigator knows the allocation of treatments.
- Blind: One of the two does not know the treatment received.
- Double-blind: Neither the patient nor the investigator knows the treatment allocation. This is the recommended method because it minimizes false results.
Types of trials in observational research:
First of all, it is important to know that an observational study is carried out at a specific time. It consists in collecting information from patients and can be done in two ways:
- Retrospective: The trial takes place after the events being studied have taken place.
- Prospective: The trial takes place before the events being studied.
The design can also be different depending on what is to be emphasized:
- Cohort study: This type of study measures the association between an exposure (e.g. smokers/non-smokers) and an event (e.g. lung cancer). Patients will be divided into groups according to their exposure (smokers are compared to non-smokers).
- Case-control study: This type of study measures the association according to the rate of exposure (smokers/non-smokers) in the cases (lung cancer patients) and in the controls (non-sufferers). The patients are divided according to whether or not they have experienced the event (e.g. ill vs. not ill).
- Cross-sectional study: For a given proportion of patients, information on the occurrence of the event and the exposure is collected simultaneously in order to obtain a numerical figure (e.g. number of people with lung cancer and number of smokers).
- Longitudinal study: In this type of study, information is collected over time (e.g. monitoring blood sugar levels in a diabetic patient).
Clinical trials are designed around respect for the patient and are essential for the continuous improvement of health products. Their procedure varies according to the objectives, whether they are experimental or purely observational.
Was this article helpful to you?
Share your thoughts and questions with the community in the comments below!
Take care!
Sources :
- Les différents types d’essais cliniques et l’encadrement législatif, Centre Hospitalier_ARRAS
- Méthodologie des essais cliniques, EUPATI
- Définitions des essais cliniques, Remede
1 comment
You will also like

What are the dangers associated with the over-the-counter sale of certain medicines?
Dec 19, 2020 • 6 comments

 Facebook
Facebook Twitter
Twitter


